“Incredible change happens in your life when you decide to take control of what you do have power over instead of craving control over what you don’t”. — Steve Maraboli Perhaps it is the thick fog of humidity engulfing the…
Allergic Reactions Related to Covid-19 Vaccinations in Allergic Patients
Joint Statement by the American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) and the American Academy of Otolaryngic Allergy (AAOA)
Allergic reactions are uncommon and anaphylaxis following vaccines is rare, occurring at a rate of approximately one per million doses for many vaccines.1,2,3 In order to prevent allergic reactions, it is important to identify individuals at increased risk by obtaining a history of allergy to previous vaccinations and vaccine components that might indicate an underlying hypersensitivity. Acute allergic reactions following vaccinations might be caused by the vaccine antigen, residual animal protein, antimicrobial agents, preservatives, stabilizers, or other vaccine components.4
Those administering vaccinations, including Pfizer-BioNTech COVID-19 vaccine and Moderna vaccine (mRNA COVID vaccines), need to be familiar with identifying and managing immediate-type allergic reactions, including anaphylaxis.
Immediate-immunoglobulin E (IgE)–mediated (type 1) immune reactions, such as anaphylaxis, usually occur within minutes of parenteral administration and involve specific IgE interactions with discrete antigens.5,6 Rapid recognition and initiation of treatment are necessary to prevent possible progression to respiratory failure or cardiovascular collapse. The CDC is recommending monitoring patients for 15-30 minutes post injection.
Epinephrine and equipment for managing an airway should be available for immediate use.7 The first and most important therapy in anaphylaxis is epinephrine. There are NO absolute contraindications to epinephrine in the setting of anaphylaxis. All anaphylactic reactions should be managed immediately with IM epinephrine as the first line treatment.
A history of severe allergic reactions not related to vaccines or injectable medications—such as allergies to food, pet, venom, environmental, or latex—is not a contraindication, and these patients can still get vaccinated. People with a history of allergies to oral medications or a family history of severe allergic reactions, or who might have a milder allergy to vaccines (no anaphylaxis) — may also still get vaccinated.8
In general, a history of a severe allergic reaction to a vaccine should be considered a contraindication to additional doses of the same vaccine.9 If a patient experiences a severe allergic reaction after getting the first dose of the mRNA COVID-19 vaccine, they should not get the second shot and referral for evaluation to possibly determine the component responsible, before making decisions regarding administration of the additional doses of the same vaccine or other vaccines that have the same components should be considered.
The recommendations below are based on best knowledge to date but may change at any time, pending new information and further guidance from the CDC or FDA.
- Individuals with common allergies to medications, foods, inhalants, venoms, and latex are no more likely than the general public to have an allergic reaction to the mRNA COVID-19 vaccines.
- The mRNA COVID-19 vaccines should be administered in a healthcare setting where anaphylaxis can be recognized and treated. Vaccination providers should have appropriate medications and equipment—such as epinephrine and equipment for managing an airway. All anaphylactic reactions should be managed immediately with IM epinephrine as the first line treatment.
- All people who receive a mRNA COVID-19 vaccine should be monitored on-site after receiving their dose. People with a history of severe allergic reactions should be monitored for 30 minutes after getting the vaccine. All others should be monitored for 15 minutes after getting the vaccine.
- According to the CDC, if a patient has a severe allergic reaction after getting the first shot, they should not receive the second shot. Additionally, the CDC notes patients who experience a severe allergic reaction should receive a referral for evaluation to possibly determine the component responsible for the reaction.
- The mRNA COVID-19 vaccines should not be administered to individuals with a known history of a severe allergic reaction to any component of the vaccine. The specific vaccine component causing the anaphylaxis has not been identified. Polyethylene glycol (PEG) is one of the vaccine’s ingredients and has been known to cause anaphylaxis.10,11,12 PEG is used as an inactive ingredient in some drugs. PEG is also used as a bowel prep for colonoscopy procedures and as a laxative.
- Data related to risk in individuals with a history of allergic reactions to other previous vaccinations and/or idiopathic anaphylaxis is very limited and evolving. A clinical decision to administer either of the COVID-19 vaccines should be undertaken by the physician or other provider administering the vaccine using their professional judgment and in consultation with the patient, balancing the benefits and risks associated with taking the vaccine.
- Recommendations are to defer vaccination for 90 days after receiving convalescent plasma or monoclonal antibody treatment for COVID-19.
Coadministration with Other Vaccines
Given the lack of data on the safety and efficacy of mRNA COVID-19 vaccines administered simultaneously with other vaccines, the mRNA COVID-19 vaccine series should be administered alone, with a minimum interval of 14 days before and after administration with any other vaccines.8 Allergen immunotherapy (SCIT, SLIT-tablets, and SLIT-aqueous) as well as biologics, such as omalizumab and dupilumab, are not considered vaccines and do not need to be held for 14 days before and 14 days after mRNA COVID-19 vaccines. Expert opinion recommends not administering subcutaneous immunotherapy injections or biologics on the same day as the mRNA vaccine and no dose adjustment for sublingual forms (both tablets and aqueous) of immunotherapy.
Immunodeficiency
The mRNA COVID-19 vaccines are not live vaccines and thus can be administered to immunocompromised patients. Providers should inform these immunocompromised patients of the possibility of a diminished immune response to the mRNA COVID-19 vaccine.
Biologics/monoclonal Antibodies
Dupilumab has several indications for use, most notable among which for otolaryngologists is as an add-on maintenance treatment in adults with inadequately controlled chronic rhinosinusitis with nasal polyposis (CRSwNP).13 According to its package insert, the use of live vaccines should be avoided with dupilumab. Dupilumab has not been studied with live vaccines. The two currently FDA approved COVID-19 vaccinations are both non-live, mRNA vaccines. There are currently no live-attenuated virus vaccines in clinical trials.14
A phase 2 DBPC study assessing the immune response to non-live vaccines in atopic dermatitis adults on dupilumab showed that antibody responses to both meningococcal polysaccharide vaccine and tetanus vaccine were similar in both placebo-treated and dupilumab-treated subjects.
Omalizumab similarly has several indications for use, including as an add-on maintenance treatment for nasal polyps in adult patients 18 years of age and older with inadequate response to nasal corticosteroids.
Dupilumab and omalizumab are classified as immunomodulator medications (medications that act by directly changing the behavior of the immune system).15 There is no evidence that dupilumab nor omalizumab have immunosuppressive effects.
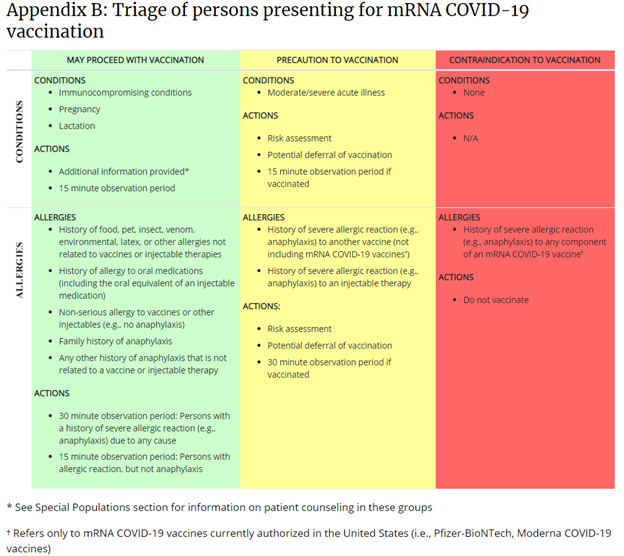
This table is published on the CDC’s webpage, “Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States.”
For more information and updates, please also visit the CDC’s webpage, “COVID-19 Vaccines and Severe Allergic Reactions.”
1 Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112(4):815-820. DOI: 10.1542/peds.112.4.815
2 McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016; 137(3):868-878.
3 Dreskin et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organization Journal 2016; 9:32.
4 Grabenstein JD. Clinical management of hypersensitivities to vaccine components. Hospital Pharmacy. 1997;32:77-87.
5 Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477-480.e471-442. DOI: 10.1016/j.jaci.2010.06.022
6 Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25-43. DOI: 10.1016/j.jaci.2012.04.003
7 Kroger A, Atkinson W, Pickering L. General immunization practices. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 6th ed. China: Elsevier Saunders; 2013:88-111.
8 https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html accessed 12/22/2020
9 Wood RA, Berger M, Dreskin SC, et al. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics. 2008;122(3):e771-777. DOI: 10.1542/peds.2008-1002.
10 Sellaturay P, Nasser S, Ewan P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J Allergy Clin Immunol Pract. 2020 Oct 1:S2213-2198(20)31007-2. doi: 10.1016/j.jaip.2020.09.029. Epub ahead of print. PMID: 33011299.
11 Stone CA, Liu Y, et al. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J Allergy Clin Immunol Pract. 2019; 7(5): 1533–1540.
12 Wylon, K., Dölle, S. & Worm, M. Polyethylene glycol as a cause of anaphylaxis. J Allergy Asthma Clin Immunol. 12, 67 (2016).
13 Dupixent (dupilumab) Prescribing Information, Regeneron and Sanofi-Genzyme, 2020.
14 Chung Y, Beiss V, Fiering S, et al. COVID-19 Vaccine Frontrunners and Their Nanotechonology Design. 2020; 14(10): 12522-12537.
15 www.aaaai.org/conditions-and-treatments/drug-guide/immunomodulator-allergy